When, two years ago, the FDA reviewed the Parkinson’s drug, Nuplazid, lead medical reviewer Dr. Paul Andreason issued grave warnings about the potential risks. During the review process, he observed that those taking the actual medication were suffering extreme side-effects (including death) twice as much as those taking placebos. In addition, he … [Read more...]
Nuplazid Deaths
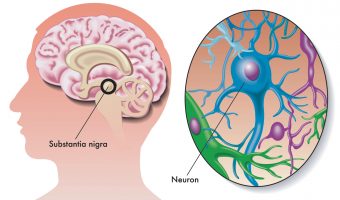
Nuplazid (pimavanserin) is a new-generation Parkinson’s disease drug, introduced in April 2016. The U.S. Food and Drug Administration (FDA) has already received at least 5,735 adverse event reports including 712 deaths linked to Nuplazid in the short time that it has been on the market. The drug is made by Acadia Pharmaceuticals and is an atypical … [Read more...]

Recent Comments