Summary
- Company Announcement Date:
- April 23, 2025
- FDA Publish Date:
- April 24, 2025
- Product Type:
- Animal & Veterinary
- Reason for Announcement:
-
Recall Reason Description
Presence of particulate matter
- Company Name:
- Cronus Pharma LLC
- Brand Name:
-
Brand Name(s)
Dexased™ and Dexmedvet™
- Product Description:
-
Product Description
Dexmedetomidine Hydrochloride Injection 0.5 mg/mL
Company Announcement
East Brunswick, NJ, April 23, 2025 – CRONUS PHARMA LLC, on behalf of Cronus Pharma Specialties, Hyderabad, India is issuing a voluntary recall involving one batch comprising two distinct lots: Dexased™ (Aspen) and Dexmedvet™ (Cronus Pharma LLC). Both products contain Dexmedetomidine Hydrochloride Injection 0.5 mg/mL, which is a prescription product in the United States used as a sedative and analgesic in dogs and cats to facilitate clinical examinations, clinical procedures, minor surgical procedures, and minor dental procedures. This product is also indicated for use as a preanesthetic to general anesthesia in dogs and cats.
The recall is being conducted as a precautionary measure due to the presence of visible particulate matter (described as crystal-like particles, white floating matter, or precipitates) observed in the solution.
Crystal-like particles/White stuff floating/ Precipitate was observed during visual inspection of control samples for the following batches:
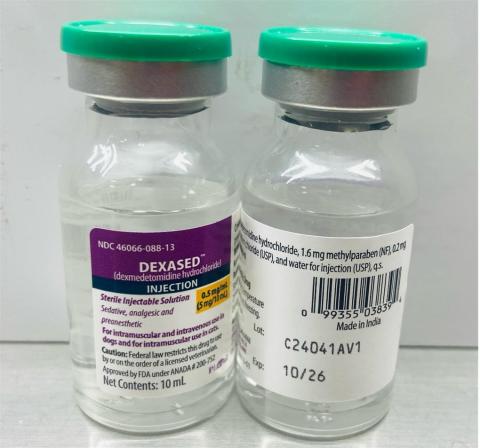
- Dexased™: Dexmedetomidine Hydrochloride Injection 0.5mg/ml (Aspen), NDC No: 46066-088-13, Batch No: C24041AV1, Mfg. Date: Nov 2024, Exp. Date: Oct 2026.
- Distribution Dates: December 2024 to 04-April-2025
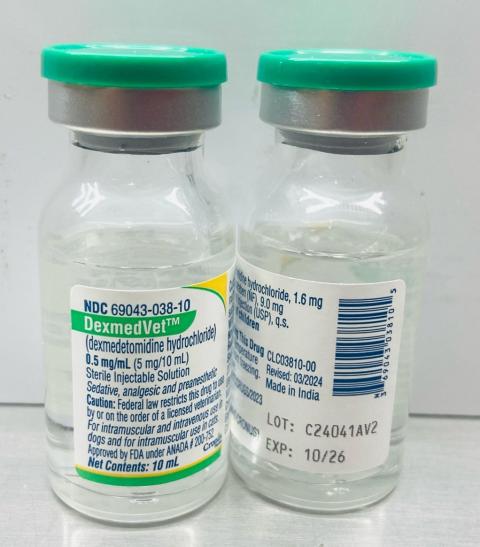
- DexmedVet™: Dexmedetomidine Hydrochloride Injection 0.5mg/ml (Cronus Pharma LLC), NDC No: 69043-038-10, Batch No: C24041AV2, Mfg. Date: Nov 2024, Exp. Date: Oct 2026.
- Distribution Dates: March 2025 to 04-April-2025
A photo of the vial of each lot number that is part of the recall can be found at the end of this press release. The lot number (LOT) and expiry date (EXP) are located on the bottom right portion of the bottle label.
Administration of an injectable product that contains particulate matter may result in serious adverse events. Potential complications include inflammation, granuloma, fibrosis, and blockage of blood vessels in the heart, lungs or brain which can cause stroke or life-threatening blood clot events, including death. The overall risk depends on factors such as the route of injection, the composition of the particulate matter, and patient comorbidities.
Some possible signs and symptoms of an adverse event include pain, weakness, swelling, paralysis, fever, labored or fast breathing, vomiting, decreased activity level, vocalization, or loss of consciousness. If you observe any of these or any other concerning signs in an animal that may have been administered this product, please contact a veterinarian as soon as possible.
Dexmedetomidine Hydrochloride Injection 0.5 mg/mL is indicated for use as a sedative and analgesic in dogs and cats.
Customers who have received Dexased™ (Aspen ) & DexmedVet™ (Cronus Pharma LLC) from the lots (C24041AV1 and C24041AV2) being recalled, should stop using the products and refer to your recall letter for product return instructions. Cronus Pharma LLC is working with its distributor partners to ensure that unused product is no longer in distribution or with customers. We are notifying our distributors and customers directly and are arranging for the return of the recalled product.
Consumers with technical questions regarding this recall should call 1-844-227-6687, Fax: 732-647-1272 and email to pv@cronuspharmausa.com (Monday through Friday 9 a.m. – 5 p.m. CDT). Customers who need to arrange return of product should contact their point of purchase for details.
Adverse reactions or quality problems experienced with the use of this product may be reported to the FDA at 1-888-FDA-VETS or online at http://www.FDA.gov/reportanimalae.
This recall is being made with the knowledge of the Food and Drug Administration.
The health and well-being of animals is the foremost priority at Cronus Pharma LLC. We place the utmost emphasis on product quality at every step in the manufacturing and supply chain process.
Company Contact Information
Product Photos








Leave a Reply